There have been 2 deaths in Americans who had monkeypox. Both were said to be severely immunocompromised.
Sept. 12, 2022, 12:33 AM EDT / Updated Sept. 13, 2022, 12:20 PM EDT
The Los Angeles County health department on Monday confirmed the first death from monkeypox in the U.S.
In a statement, the department said the patient was severely immunocompromised and had been hospitalized, but did not provide further details.
The Centers for Disease Control and Prevention has confirmed the death as well, the department said.
In Texas, health officials are still investigating the death of a person with monkeypox. When the death was reported on August 29, the Texas Department of State Health Services said the patient was diagnosed with monkeypox in the Houston area and was "severely immunocompromised." But it's not yet known whether monkeypox was the cause of death.
There was also one reported death in a Hollywood makeup artist 9 days after receiving a monkeypox vaccine injection.
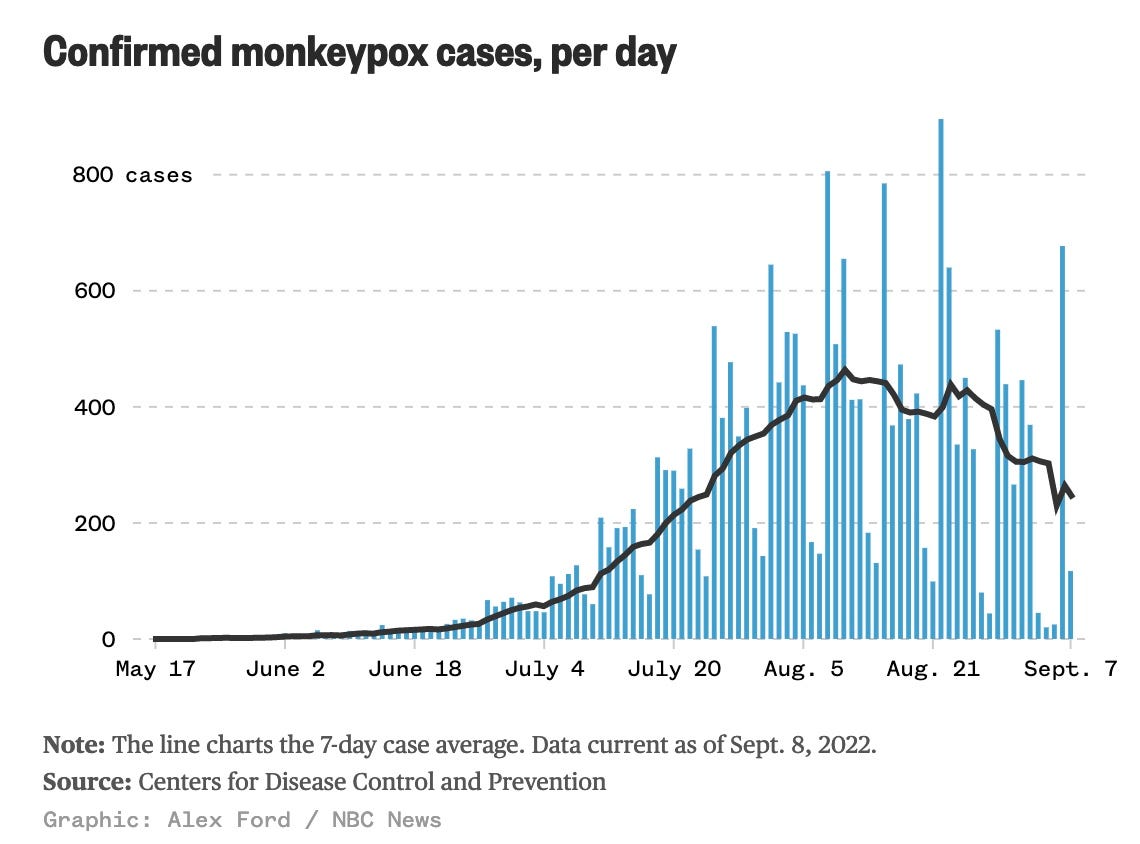
Fortunately, monkeypox cases have been steadily dropping over the past 3 weeks.
As Demand for the Monkeypox Vaccine Stalls, Outreach Goes Hyperlocal
Federal health officials working to stem the monkeypox outbreak are shifting tactics in their immunization campaign as interest in the vaccine wanes and gaps in getting the shot to communities of color persist.
Earlier this summer, eager people snapped up vaccination appointments in cities from New York to Los Angeles. But a POLITICO review of a Biden administration pilot program that began last month to offer shots at large events found that supply outpaced demand, a trend mirrored nationwide as vaccine uptake has slowed.
Now the administration says it’s widening the net, creating another pilot to send vaccines to smaller venues and clinics.
I have pointed out that vaccine did not prevent monkeypox in monkeys. It did generate antibody levels…but without a correlate of protection antibody levels don’t mean much. All vaccine candidates generate antibodies of some kind. In an apparent attempt to show the vaccine “works”, the manufacturer has released some unpublished data, according to CIDRAP at the University of Minnesota:
In contrast to a recent Dutch preprint that cast doubt on the efficacy of Bavarian Nordic's Jynneos (modified vaccinia Ankara [MVA]) vaccine to produce significant neutralizing antibodies to monkeypox, the company has released its own preprint study showing that single and two-dose Jynneos vaccinations administered subcutaneously induced durable neutralizing antibody responses in healthy volunteers.
Results were comparable to older-generation replicating smallpox vaccines.
But here’s the thing: The CDC gave the vaccine to 1000-1600 healthcare workers in an area of the Congo where many were expected to be exposed. Vaccinations began in 2017 and were completed by 2020 or earlier.
The 2017 clinicaltrials.gov report of this trial says it will enroll 1600 healthcare workers. The 2018 publication describing the logistics of the trial says the target enrollment was 1000. This publication also states that “16 weeks were required for initial enrollment of participants.” It further describes its methodology:
Serologic monitoring of study participants will continue at roughly 6-month intervals until the two-year timepoint is reached. Until that time, the study team will continue to collect information pertaining to participants’ occupational exposures to MPX cases—presumptive and confirmed—, MPX infection status, and possible vaccine adverse events. Finally, as a surrogate measure for immunization efficacy, we will measure participants’ serum orthopoxvirus antibody titers and will assess whether specific participant characteristics (e.g., age, sex, prior smallpox immunization) are associated with more enduring antibody levels…
Understanding the performance parameters of IMVAMUNE [one of the many earlier names of Jynneos—Nass] under conditions of natural orthopoxvirus transmission will build confidence in its use as a preventive measure for many populations potentially at risk, including HCWs, and, in the future, perhaps hunters or other groups with elevated risk.
Yet CDC has breathed not a word of the results of this extensive field trial, the only trial of efficacy—ever—for this vaccine.
If the results were positive, don’t you think CDC would be shouting them from the rooftops as it seeks more and more recipients for its monkey business vaccine?
And now they want our children to join the experiment. Or maybe it is not an experiment and the federal officials know exactly what they are doing.



















No comments:
Post a Comment