Tricks used by CDC to minimize vaccine injury rates
CDC has become very enamoured of the VAERS database for Covid shots. This is very curious, because CDC used to claim this database was only good for identifying signals, and not for calculating the rates of adverse vaccine reactions. I have previously cited the CDC's own VAERS website, which says you cannot calculate adverse event rates using VAERS (because of the high but unknown extent of underreporting).
VAERS is a 30 year old federal vaccine safety surveillance system. It collects data supplied voluntarily by patients, doctors, and relatives regarding adverse events occurring after vaccinations.
CDC created the V-Safe system as a new way to collect adverse event data on Covid vaccines. V-safe has enrolled several million people receiving Covid vaccinations, who receive text messages asking them to report certain adverse events. For some reason, CDC does not collect the answers directly, but instead the reports go to the Oracle company. Oracle aggregates the data and sends the results weekly to CDC.
Inexplicably, CDC instructed Oracle to identify serious adverse events in the reports it receives, and send them to VAERS. This causes the serious V-Safe reports to be doubly reported. Taking data reported to one database and adding it to a different database seems to me to serve the purpose of confusing the statistical analysis of the data, and making it impossible to estimate how the reporting rate to VAERS compares with the actual occurrence rate of adverse events, and with previous VAERS reporting rates.
Past studies of VAERS underreporting (prior to the V-Safe additions) have estimated that only between 1 in 13 and 1 in 100 adverse events gets reported to the system. See:
https://childrenshealthdefense.org/defender/rfk-jr-david-kessler-covid-vaccine-vaers/
But now CDC suddenly claims that "VAERS is the nation’s early warning system for vaccine safety," according to slides 9 and 15 of CDC's presentation. The identical slide seems to have been repeated for emphasis in the presentation to CDC's Advisory Committee for Immunization Practices by CDC's Tom Shimabukuro on June 23.
The missing CDC databases
While VAERS is useful, but should not be used to calculate adverse event rates, CDC has a number of other databases that have statistical validity, which VAERS lacks. In fact, CDC claimed to be using many such databases to assess the safety of Covid vaccines. Here is a list that CDC's Immunization Center Director presented last December:

If the CDC seriously wanted to assess the actual rate of myocarditis following Covid vaccinations, an excellent place to start would be the military's Defense Medical Surveillance System (DMSS). It is listed as one of the surveillance systems CDC would be using in the graphic above.
Every military servicemember's vaccinations, outpatient visits and hospitalizations are included in this database, so if CDC told us what the DMSS data showed, there would be no need for guesswork regarding the actual rates of adverse events in US soldiers. But instead, CDC keeps these more valid data hidden, and falls back on VAERS, improperly calculating "observed vs expected rates" from a database with an acknowledged huge rate of underreporting. The only explanation for why this is done is to reduce the apparent adverse event rate.
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVID-Shimabukuro-508.pdf
Preliminary myocarditis/pericarditis reports to VAERS following dose 2 mRNA
COVID-19 vaccination, Exp. vs. Obs. using 21 day- risk window (data thru Jun 11, 2021)
* Assumes a 21-day post-vaccination observation window (i.e., symptom onset from day of vaccination through Day 20 after vaccination)
† Based on Gubernot et al. U.S. Population-Based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine. 2021 May 14:S0264-
410X(21)00578-8. Expected counts among females 12–29 years adjusted for lower prevalence relative to males by factor of 1.7 (Fairweather, D. et al, Curr Probl Cardiol. 2013;38(1):7-46).
Using the VAERS self-reported data, the rate of reported myocarditis in boys in the 12-17 year old group, after the second Pfizer shot, is about 1/15,000. These data are presented in slides 27 and 28. However, if the actual rate is 10 times greater than the reported rate, then about 1 in 1500 boys got myocarditis after their second shot. If the rate is 100 times greater than the VAERS reporting rate, then about 1 in 150 boys aged 12 through 17 got myocarditis after their second shot.
But we also need to consider those (about 20% of the total) who got myocarditis after their first Pfizer shot. If we add them to the boys who developed myocarditis after their second shot, then our estimated chance of boys aged 12-17 getting myocarditis is in the range of about 1 in 120 to 1 in 1200.
The younger you are, the more likely you are to get myocarditis after a Covid shot.
And then there is the fact that if you are going to compare the expected rate with the observed rate of myocarditis post-vaccination, you need to be using comparable groups to compare the rates. But the VAERS database is not a database that can be used quantitatively, since we do not know much about the characteristics of the VAERS-reporting population.
Finally, there is a backload of VAERS reports that have not been input into the system. Some say the backload is up to 2 months of data--we really do not know how much missing data there is.
Now, I really don't want to use VAERS (+/- V-Safe) data to come up with these estimates. I would much prefer to give you real numbers. But CDC is hiding the real numbers from all those databases they listed above, and pretending that VAERS, V-Safe and a tiny cohort from the VSD is all they've got.
Hiding the actual adverse event rates while using psychological, financial, educational and other methods to pressure people into taking an experimental vaccine ought to be a crime. It probably is a crime, and the US public health agencies are co-conspirators.
UPDATE June 29: I just listened to Dr. Robert Malone's interview with Del Bigtree last week. Malone is the MD, PhD who first began using mRNA technology in vaccines. He pointed out that myocarditis may be causing heart attacks in older age groups... where the diagnosis may be incorrectly attributed to coronary artery disease, rather than to inflammation resulting from a Covid-19 vaccine.
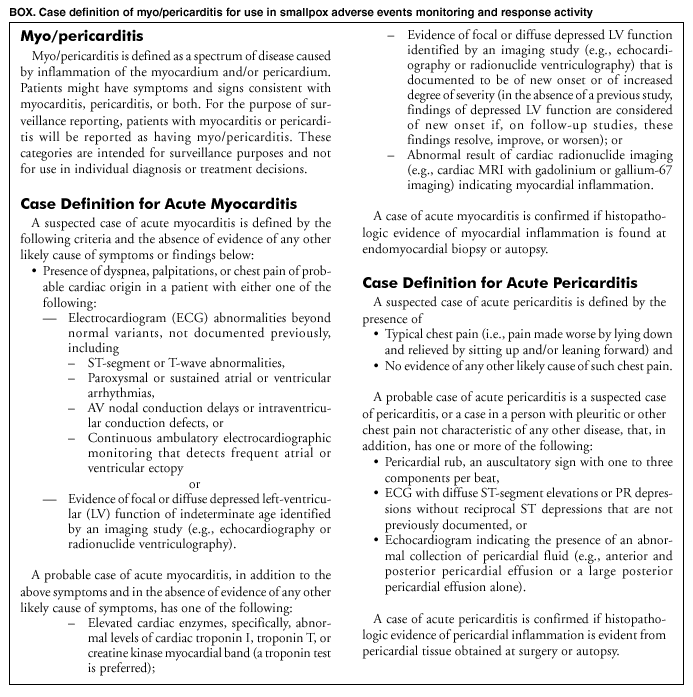
It is important to remember that smallpox vaccinations were stopped in the US decades ago because of high adverse reaction rates. Especially cardiac reactions. Myo/pericarditis as a consequence of vaccination is not new. It has been observed for many decades.
There have been no natural smallpox cases in the world since 1977.
However, US smallpox vaccinations were restarted in 2003, congruent with the Bush administration program to demonize Saddam Hussain over the risk of biological warfare. After 50,000 smallpox vaccinations were given there were many cardiac events and some deaths reported, and the civilian vaccinations stopped, as the medical personnel targeted for them refused to accept them.
But the smallpox vaccinations were continued for the military, where servicemembers continued to have high adverse reaction rates. Malone referenced a 2015 paper written by military physicians (the first 3 authors are people whose work I respect) documenting high rates (214 times the baseline rate) of myocarditis-pericarditis in those being vaccinated.
Below is the abstract from this paper. And here is one of at least 8 reports on smallpox vaccine and the resulting cardiac complications, published by CDC, in its 2003 Morbidity and Mortality Weekly Reports.
What CDC appears to have learned from the 2003 smallpox program is that it needed to cover up the severe adverse reactions, in order to keep up the vaccinations.
. 2015 Mar 20;10(3):e0118283.
A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination
doi: 10.1371/journal.pone.0118283. eCollection 2015.Renata J M Engler 1, Michael R Nelson 2, Limone C Collins Jr 3, Christina Spooner 3, Brian A Hemann 4, Barnett T Gibbs 4, J Edwin Atwood 4, Robin S Howard 5, Audrey S Chang 5, Daniel L Cruser 6, Daniel G Gates 7, Marina N Vernalis 8, Marguerite S Lengkeek 9, Bruce M McClenathan 10, Allan S Jaffe 11, Leslie T Cooper 11, Steve Black 12, Christopher Carlson 13, Christopher Wilson 14, Robert L Davis 15 Abstract
Background: Although myocarditis/pericarditis (MP) has been identified as an adverse event following smallpox vaccine (SPX), the prospective incidence of this reaction and new onset cardiac symptoms, including possible subclinical injury, has not been prospectively defined.
Purpose: The study's primary objective was to determine the prospective incidence of new onset cardiac symptoms, clinical and possible subclinical MP in temporal association with immunization.
Methods: New onset cardiac symptoms, clinical MP and cardiac specific troponin T (cTnT) elevations following SPX (above individual baseline values) were measured in a multi-center prospective, active surveillance cohort study of healthy subjects receiving either smallpox vaccine or trivalent influenza vaccine (TIV).
Results: New onset chest pain, dyspnea, and/or palpitations occurred in 10.6% of SPX-vaccinees and 2.6% of TIV-vaccinees within 30 days of immunization (relative risk (RR) 4.0, 95% CI: 1.7-9.3). Among the 1081 SPX-vaccinees with complete follow-up, 4 Caucasian males were diagnosed with probable myocarditis and 1 female with suspected pericarditis. This indicates a post-SPX incidence rate more than 200-times higher than the pre-SPX background population surveillance rate of myocarditis/pericarditis (RR 214, 95% CI 65-558). Additionally, 31 SPX-vaccinees without specific cardiac symptoms were found to have over 2-fold increases in cTnT (>99th percentile) from baseline (pre-SPX) during the window of risk for clinical myocarditis/pericarditis and meeting a proposed case definition for possible subclinical myocarditis. This rate is 60-times higher than the incidence rate of overt clinical cases. No clinical or possible subclinical myocarditis cases were identified in the TIV-vaccinated group.
Conclusions: Passive surveillance significantly underestimates the true incidence of myocarditis/pericarditis after smallpox immunization. Evidence of subclinical transient cardiac muscle injury post-vaccinia immunization is a finding that requires further study to include long-term outcomes surveillance. Active safety surveillance is needed to identify adverse events that are not well understood or previously recognized.