1. Why did all of the vaccines chosen for Operation Warp Speed use novel technologies, unused in any routine vaccines? (Yes, a new Ebola vaccine uses an adenovirus vector, and a resurfaced military-only adenovirus vaccine uses one too--but that's all.) Why wasn't a method with a proven track record chosen for the largest vaccine program in the history of the world?
2. Why did all 4 of the initial vaccines selected for development in Operation Warp Speed (Moderna, Pfizer, J and J/Janssen and Astra-Zeneca) rely on using RNA or DNA?
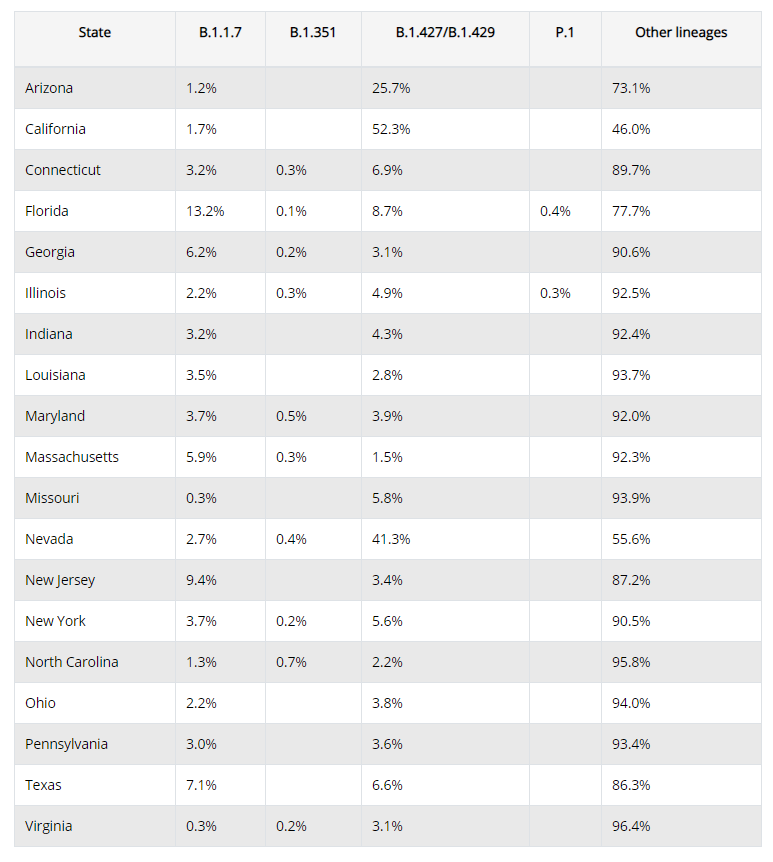
3. Why did all 4 vaccines rely on the "S" or Spike protein as their only antigen, despite accumulating evidence that multiple segments on it might stimulate autoimmunity? And despite evidence that the spike protein itself explained some or most of Covid's toxic effects on human tissues?
4. Why have government and industry authorized and unleashed 340 different lab tests for Covid in the US, only one of which has been FDA-approved, and none of which are reliably accurate?
5. Why are government and many experts lying about the fact that recovered people should be vaccinated, when vaccinating them puts them at higher risk of immune adverse reactions?
6. Why are government and experts lying to claim that vaccine-induced immunity will be stronger, more robust and long-lasting than natural immunity, when this is an outright falsehood for which there is zero evidence? Unless they have lied that the mRNA and DNA will be destroyed in hours or a few days, and instead the vaccinated will become long-term Spike factories?
7. Why do governments and experts contradict the last lie with another lie, which claims that new variants will escape natural and vaccine-induced immunity, so that we can expect more shots every 6-12 months in future, tweaked to the current variants?
8. Why don't they tell the truth, that variants with multiple spike mutations can defeat the current vaccines, but are much less likely to defeat natural immunity, which depends on many other immune epitopes, not just the spike.
9. Why do leaders like Angela Merkel repeat in unison, "[We] will not be able to rest easy until the entire world receives access to ample supplies of vaccines against the coronavirus," Most of Africa had a tiny fraction of the deaths seen in the Western Hemisphere and Europe. Why do they need supplies of vaccine? Why do these otherwise heartless leaders want so badly to share their vaccines?
Why do they flip-flop back and forth about how the vaccines will or won't protect against viral variants? For example, the NY Times: As Virus Variants Spread, 'No One is Safe Until Everyone is Safe.'
Here is more of the same propaganda: "Unless we vaccinate the world, we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them."
—Gregg Gonsalves, Yale University
Well, if we vaccinate the whole world, even children for whom the vaccine is unnecessary, what about all the cats, ferrets, minks and mice that are still susceptible? There is no possibility of "zero covid" --like the flu, this virus will be with us until it decides to leave. We cannot disappear it. Yet the meme persists.
10. Why, if our world leaders actually cared about saving lives, have they so aggressively suppressed HCQ and ivermectin for early, effective Covid treatment? Note that the EMA advised against ivermectin one week ago, as the FDA did in February--neither actually explaining what evidence they reviewed and how they drew their conclusion.
11. Why don't they tell the truth, that Vitamin D deficiency is a major risk factor for serious illness? And that a few pennies' worth of oral vitamin D taken weekly or even monthly is probably your best insurance from dying from Covid? (The UK has started prescribing Vitamin D for high-risk individuals.)
12. Why are outlets like the Washington Post publishing lies like "Vaccine passports have little to do with international travel " when several airlines are already trialing them?
13. Why is it so important to roll out vaccine passports when no standards for them even exist yet, and the privacy issue is a real problem? They were voted in by the European Parliament one week ago, and have been rolled out in Israel and NY state (the Excelsior pass) for events.
14. Why do officials claim these passports will be 'free,' when the cost to build, provide interoperability and maintain the new systems will be many billions of taxpayer dollars?
15. Why are the US, Israel and western Europe rushing to duplicate the practices of the Nazis (Your papers please!")?
16. Why did FDA permit the vaccine companies to design their clinical trials with loose criteria to identify cases (1 or 2 mild symptoms and a positive PCR test, which used an excessive cycle threshold of 40, leading to false positives)?
17. Why did FDA then allow the trials to essentially stop as soon as the vaccines received an EUA: which is when FDA approved the companies' request to offer vaccine to all the placebo subjects, thus making it impossible to obtain long-term safety data using the placebo group for comparison?
18. Why has the FDA allowed numerous entities and so-called fact-checking sites to claim the current Covid vaccines, issued under EUA, are "safe and effective" or "approved" when they do not meet either standard, and it is FDA's responsibility to quash such false claims immediately?
19. Why, over the past several weeks, have the FDA, the EMA and now the WHO issued warning against the use of ivermectin for Covid, when the scientific evidence is overwhelmingly in favor of its considerable benefits? Here is a brief summary of the extensive data (for all the Covid drugs) that can be found at c19study.com. Below is a compilation of studies on ivermectin:
20. Why did the clinical trials for all the Covid vaccines fail to
include pregnant women, yet pregnant women are being
encouraged to be vaccinated, despite many case reports of
miscarriages following vaccinations?
21. Why have CDC and FDA failed to share the safety data
they have from the Vaccine Safety Datalink, the Genesis database,
the V-safe data, the Defense Medical Surveillance System, the VA
database, BEST and others? Why do they pretend they cannot link
vaccinees with their medical records, when they have extensive online
data from every vaccinee, or could get it from state databases?
22. Why has CDC, with 10,000 employees and a huge budget,
not made the assessment of safety its number 1 priority, when it is
spending big to convince and coerce the entire population
to partake in a grand Covid vaccine experiment?
23. Why are we being threatened with inability to travel and enjoy
other "privileges" without vaccination and the vaccine passport?
It seems the idea is to impose both before the public realizes
that herd immunity is nearly here, the vaccines don't work well
and are much more dangerous than billed.
24. Why, if the vaccines worked well, are the vaccinated encouraged
to shun and shame the unvaccinated into participating in
the experiment?
25. Why, in the past week, has it suddenly become mainstream
to suspect the pandemic is due to a lab leak, when for the past year
March 2020). What is the purpose of this change in the narrative?
resolution affirming the important role of Vitamin D in Covid protection
(and protection from other infections)? Why are our so-called public
health agencies turning public health on its head, encouraging
useless drugs and experimenting on us, while withholding safe and
effective vitamins, OTC treatments, and suppressing the use of licensed drugs
that are highly effective for Covid?
27. Why have most Americans failed to see what is in front of their faces?