Turkey started testing early, with rapid results. They did case-finding and quarantines, and they used hydroxychloroquine as soon as someone got sick. Mortality in a premier hospital in Turkey for Covid-19 is under 1%. Mortality in New York hospitals for Covid-19 cases has been reported here and here as 20%.
The BBC goes into depth on how Turkey handled the pandemic. Definitely worth a read.
Saturday, May 30, 2020
Make your own mask, as good as an N95, and washable too/ Consumerlab.com
The following discussion comes straight from consumerlab, which is a subscription service. This is the best information I have found for producing a washable, highly effective mask that can protect both the user and everyone else nearby--Meryl
Here is their August 2020 update.
Question:
How can I make a mask that is as good as a surgical mask or an N-95 mask?
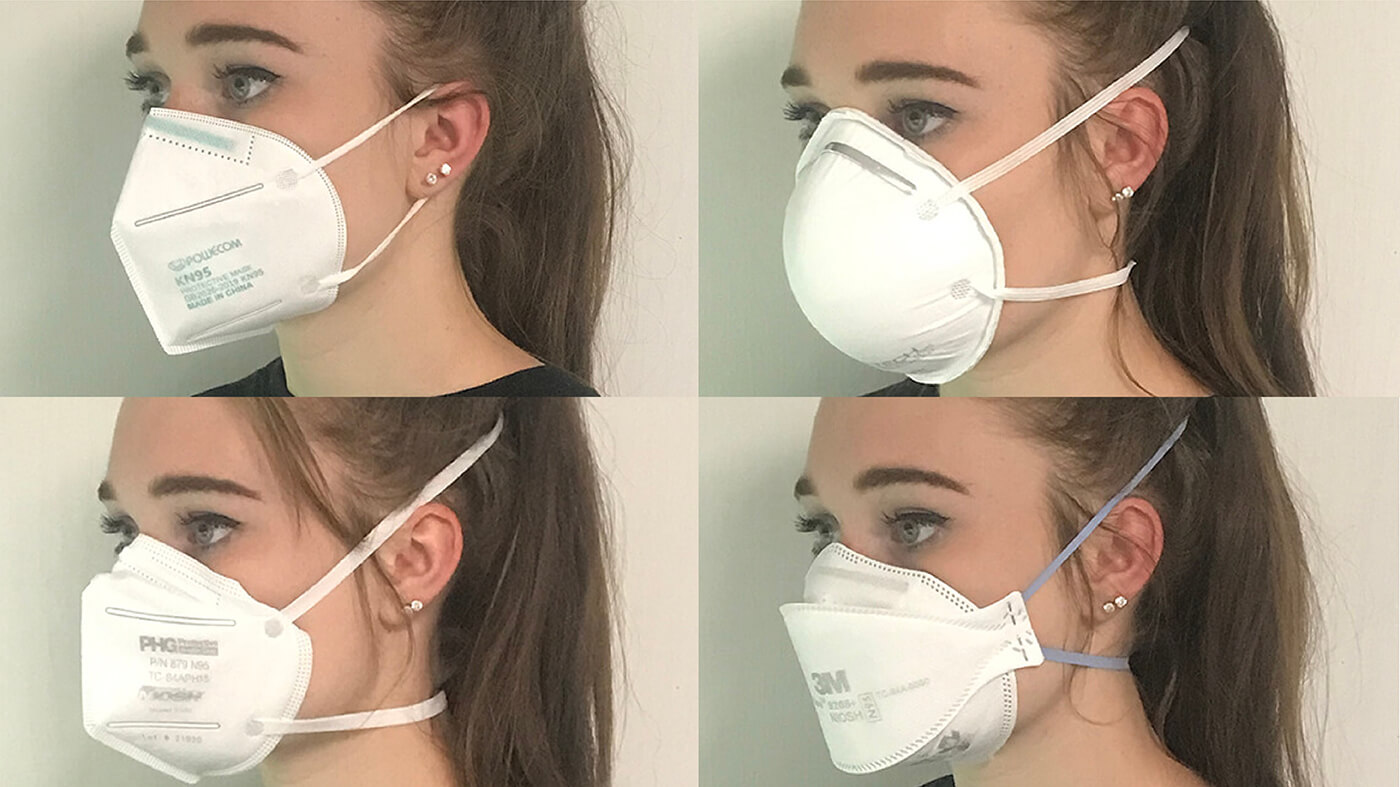
Answer:
If made with the right household materials, you can create a mask that may be as effective as a medical mask or even an N-95 respirator, according to several laboratory studies. Masks can be used alone or, for increased protection, particularly for the eyes, with a face shield.
How materials compare in blocking coronavirus
The first study, from the University of Illinois at Urbana-Champaign, found that many household fabrics can be as effective as the material in surgical masks for blocking droplets the size of the COVID-19 (SARS-CoV-2) coronavirus. The blocking efficiency of a commercial medical mask was found to be 96.3%, while the blocking efficiency of a used dish cloth (85% polyester and 15% nylon) was slightly better -- 97.9%. In addition, most household fabrics were more breathable than the material in a medical mask. The dish cloth, for example, was twice as breathable as the medical mask (Aydin, medRxiv 2020 --preprint). (See the CDC website to learn how to make a cloth face covering.)
A study at the University of Chicago and Argonne National Laboratory found that tightly woven, high-thread count cotton (600 thread-per-inch (TPI) sheet by Wamsutta) was more effective in filtering large droplets (similar to larger-sized SARS-CoV-2 droplets) than loosely woven cotton with a lower thread count (quilters cotton, 80 TPI), while fabrics with an electrostatic charge (such as silk and chiffon) were best for blocking aerosols -- the smaller sized droplets that remain suspended in air for extended amounts of time. Using layers of both fabrics, together, was most effective for blocking both large and small droplets. For example, two layers of 600 TPI cotton fabric had a large particle and small particle blocking efficacy of 99.5% and 82%, respectively, but one layer of 600 TPI cotton combined with two layers of chiffon (90% polyester, 10% spandex from Jo-Ann Stores) had a large particle and small particle blocking efficacy of 99.2% and 97% -- which is nearly as good as a properly-fitted N95 mask for blocking large particles and better than the N-95 with respect to small particles, of which only 85% are blocked by an N-95 mask). The researchers also found that small holes or leaks around the edges of the fabrics could decrease the blocking efficacy by 50% or more, and emphasized the importance of a good fit (snug and without gaps) (Konda, ACS Nano 2020). [Note: An illustration in the study shows the electrostatic layer of fabric as the inner layer when fabrics were combined. However, ConsumerLab contacted the author of the study who suggested that electrostatic fabric (such as chiffon) may be best used as the outer layer of the mask to avoid humidity from the nose or mouth, which could interfere with the electrostatic properties, but emphasized that was his suggestion, not something that was tested in the study.]
How to reduce air leakage around a mask
A way to reduce air leaks was suggested by a study, at Northeastern University in Boston, which showed that pulling an 8 to 10-inch tube of nylon (cut from a queen-sized nylon stocking) down over a regular mask and to the top of the neck. This significantly prevented air leakage around the mask and improved particle filtration efficiency, making the combined masking nearly as effective as an N-95 respirator which, unlike a medical mask, has an electrostatic charge and is specifically designed to prevent air leakage (Mueller, medRxiv 2020 --preprint; Godoy, NPR.org 4/22/20).
How to clean a cloth mask
Cloth masks can be washed in a washing machine. They can also be cleaned using heat, but a washing machine is preferred.
Here is their August 2020 update.
Question:
How can I make a mask that is as good as a surgical mask or an N-95 mask?

Answer:
If made with the right household materials, you can create a mask that may be as effective as a medical mask or even an N-95 respirator, according to several laboratory studies. Masks can be used alone or, for increased protection, particularly for the eyes, with a face shield.
How materials compare in blocking coronavirus
The first study, from the University of Illinois at Urbana-Champaign, found that many household fabrics can be as effective as the material in surgical masks for blocking droplets the size of the COVID-19 (SARS-CoV-2) coronavirus. The blocking efficiency of a commercial medical mask was found to be 96.3%, while the blocking efficiency of a used dish cloth (85% polyester and 15% nylon) was slightly better -- 97.9%. In addition, most household fabrics were more breathable than the material in a medical mask. The dish cloth, for example, was twice as breathable as the medical mask (Aydin, medRxiv 2020 --preprint). (See the CDC website to learn how to make a cloth face covering.)
A study at the University of Chicago and Argonne National Laboratory found that tightly woven, high-thread count cotton (600 thread-per-inch (TPI) sheet by Wamsutta) was more effective in filtering large droplets (similar to larger-sized SARS-CoV-2 droplets) than loosely woven cotton with a lower thread count (quilters cotton, 80 TPI), while fabrics with an electrostatic charge (such as silk and chiffon) were best for blocking aerosols -- the smaller sized droplets that remain suspended in air for extended amounts of time. Using layers of both fabrics, together, was most effective for blocking both large and small droplets. For example, two layers of 600 TPI cotton fabric had a large particle and small particle blocking efficacy of 99.5% and 82%, respectively, but one layer of 600 TPI cotton combined with two layers of chiffon (90% polyester, 10% spandex from Jo-Ann Stores) had a large particle and small particle blocking efficacy of 99.2% and 97% -- which is nearly as good as a properly-fitted N95 mask for blocking large particles and better than the N-95 with respect to small particles, of which only 85% are blocked by an N-95 mask). The researchers also found that small holes or leaks around the edges of the fabrics could decrease the blocking efficacy by 50% or more, and emphasized the importance of a good fit (snug and without gaps) (Konda, ACS Nano 2020). [Note: An illustration in the study shows the electrostatic layer of fabric as the inner layer when fabrics were combined. However, ConsumerLab contacted the author of the study who suggested that electrostatic fabric (such as chiffon) may be best used as the outer layer of the mask to avoid humidity from the nose or mouth, which could interfere with the electrostatic properties, but emphasized that was his suggestion, not something that was tested in the study.]
How to reduce air leakage around a mask
A way to reduce air leaks was suggested by a study, at Northeastern University in Boston, which showed that pulling an 8 to 10-inch tube of nylon (cut from a queen-sized nylon stocking) down over a regular mask and to the top of the neck. This significantly prevented air leakage around the mask and improved particle filtration efficiency, making the combined masking nearly as effective as an N-95 respirator which, unlike a medical mask, has an electrostatic charge and is specifically designed to prevent air leakage (Mueller, medRxiv 2020 --preprint; Godoy, NPR.org 4/22/20).
How to clean a cloth mask
Cloth masks can be washed in a washing machine. They can also be cleaned using heat, but a washing machine is preferred.
Friday, May 29, 2020
Hydroxychloroquine (HCQ). Corrupt, coordinated assault managed by WHO on an inexpensive and effective treatment / Nass
I think most people have already figured out you can't trust the mass media on the subject of the chloroquine drugs and Covid-19... let's avoid the question of which subjects can they be trusted with?
I thought you could trust medical journals to a degree, but even they, whose editors are physicians, have been often unreliable on the value of chloroquines in Covid-19. How could the Lancet editors and reviewers decide to publish the very sketchy Lancet article I critiqued a few posts back? The Lancet is the world's most-read medical journal.
Today, over 140 academics and clinicians around the world have challenged the veracity of that Lancet article, and asked for it to be reviewed by an independent international group. I put up the Zenodo link for their letter at noon today, May 29, but tonight that link is gone. On May 30, link now works. Nevertheless, their letter has drawn huge attention to the questionable provenance of the Lancet paper's data, including by the Guardian and the NY Times. Why?
Because this single Lancet paper started an avalanche that ended further study of hydroxychloroquine by the WHO, and consequently some countries (Belgium, France, Italy) banned its use for treatment of Covid-19. The Jakarta Post/Reuters reported on May 27 that WHO had instructed Indonesia's health ministry to suspend the use of hydroxychloroquine, not only in clinical trials, but also for treatment of Covid-19. Indonesia, the world's 4th most populous country, had been using the drug early for all cases, independent of severity, with good results. Indonesia refused to comply.
Tony Fauci announced the same day that the drug was ineffective, and a Guardian article said that the US FDA is reconsidering its guidance permitting prescribing of hydroxychloroquine for Covid-19.
I will go further down this disturbing and fascinating rabbit hole later in this post.
Because this single Lancet paper started an avalanche that ended further study of hydroxychloroquine by the WHO, and consequently some countries (Belgium, France, Italy) banned its use for treatment of Covid-19. The Jakarta Post/Reuters reported on May 27 that WHO had instructed Indonesia's health ministry to suspend the use of hydroxychloroquine, not only in clinical trials, but also for treatment of Covid-19. Indonesia, the world's 4th most populous country, had been using the drug early for all cases, independent of severity, with good results. Indonesia refused to comply.
Tony Fauci announced the same day that the drug was ineffective, and a Guardian article said that the US FDA is reconsidering its guidance permitting prescribing of hydroxychloroquine for Covid-19.
I will go further down this disturbing and fascinating rabbit hole later in this post.
Returning to what the medical literature truly tells us about hydroxychloroquine for Covid-19, I credit the Annals of Internal Medicine (premier journal for my speciality) with an honest meta-analysis of the subject, which went online May 27, 2020.
What the meta-analysis said:
Background: Hydroxychloroquine (HCQ) and chloroquine (CQ) have antiviral effects in vitro against severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2).
Purpose: To summarize evidence about the benefits and harms of hydroxychloroquine or chloroquine for the treatment or prophylaxis of coronavirus disease 2019 (COVID-19).
Study selection: Studies in any language reporting efficacy or safety outcomes from hydroxychloroquine or chloroquine use in any setting in adults or children with suspected COVID-19 or at risk for SARS-CoV-2 infection.
Data synthesis: Four randomized controlled trials, 10 cohort studies, and 9 case series assessed treatment effects of the medications, but no studies evaluated prophylaxis. Evidence was conflicting and insufficient regarding the effect of hydroxychloroquine on such outcomes as all-cause mortality, progression to severe disease, clinical symptoms, and upper respiratory virologic clearance with antigen testing...
Limitation: There were few controlled studies, and control for confounding was inadequate in observational studies.
Conclusion: Evidence on the benefits and harms of using hydroxychloroquine or chloroquine to treat COVID-19 is very weak and conflicting.
So that is the real bottom line: the evidence on hydroxychloroquine for Covid-19 is generally poor, conflicting, and so far fails to tell us its true risks and benefits. I find it hard to believe that after 5 months and 5 million cases of Covid-19, this failure is not deliberate.
--------------
What did Didier Raoult's group just report from France? They treated over 1000 (previously unreported on) patients, and have increased the dose of HCQ to 200 mg 3x daily for up to 10 days, used it with the traditional Z-pak dose of azithromycin, and say 98.7% are cured, 4.3% had a poor clinical outcome and 0.8% died. No deaths were cardiac, all due to respiratory failure. About one sixth of their patients did not receive HCQ due to a contraindication or refusal.
--------------
What can we discern from other countries? Costa Rica is said to be the only country in Central America routinely using HCQ for early treatment. "In Costa Rica, all patients — including those with with minor symptoms or who are asymptomatic — are offered the option to take hydroxychloroquine upon their diagnosis, as long as they don’t have contraindications to the drug. Costa Rica has a low case fatality rate (1.07%), and fewer than 5% of known active coronavirus cases are currently hospitalized." But it will be reconsidering this practice as a result of the WHO's decision, according to the Tico Times. Costa Rica has fewer cases and deaths than any country in central America.
Below is an interesting compilation from Elizabeth Lee Vliet, MD, who writes, "Examples from the world data on May 18, 2020, which is updated daily, show how [some] Third-World countries are faring far better than the U.S." The countries doing well are using HCQ routinely.
Country
|
# of cases
|
# of deaths
|
Deaths/million
|
Use of HCQ
|
India
|
101,261
|
3,164
|
2.0
|
Early and prophylactic
|
Costa Rica
|
866
|
10
|
2.0
|
Early and prophylactic
|
Australia
|
7,068
|
99
|
4.0
|
Early and prophylactic
|
South Korea
|
11,078
|
263
|
5.0
|
Early and prophylactic
|
Argentina
|
8,371
|
382
|
8.0
|
Early and prophylactic
|
Turkey
|
150,593
|
4171
|
50.0
|
Early and prophylactic
|
Israel
|
16,643
|
276
|
32.0
|
Early and prophylactic use
|
Brazil
|
255,368
|
16,853
|
79.0
|
Early, some prophylactic use
|
U.S.
|
1,550,294
|
91,981
|
278.0
|
Late, in hospitalized patients
|
Now, I return to the WHO stopping the use of HCQ in its multi-nation trial of Covid-19 therapies...allegedly based on the Lancet study I reviewed here, which itself relied on a wholly opaque commercial database, "Surgisphere," owned by one of the Lancet authors, Sapan Desai. The study had a too-good-to-be-true overall death rate, inaccurate data for Australia, and no ability to verify the accuracy of the rest of its data or even identify the hospitals it came from...and the Lancet has already issued an initial correction.
James Todaro, MD shows us that the Surgisphere company had only one employee, its founder Sapan Desai, until 2-3 months ago. Yet it supposedly manages a database comprised of 671 hospitals' data? Something is very fishy. Dr. Chris Martenson provides his own take on the study, here. Australasian statistician Peter Ellis has discovered that a litany of awards Surgisphere's sister database company (Quartz Clinical) claims to have received--were won by others. And it had no online presence until last September.
Bottom line, it is almost certain that the data the Lancet paper presented from Mehra, Desai et al. were fabricated, for many statistical and logistical reasons that are discussed in the links I have provided, especially in that letter with, now, 182 signatories. Yet the Mehra, Desai et al. paper, in the world's top medical journal, was intended to be the death knell for hydroxychloroquine (and subsequently for many patients with Covid). Are you starting to see how deep the rot goes?
James Todaro, MD shows us that the Surgisphere company had only one employee, its founder Sapan Desai, until 2-3 months ago. Yet it supposedly manages a database comprised of 671 hospitals' data? Something is very fishy. Dr. Chris Martenson provides his own take on the study, here. Australasian statistician Peter Ellis has discovered that a litany of awards Surgisphere's sister database company (Quartz Clinical) claims to have received--were won by others. And it had no online presence until last September.
Bottom line, it is almost certain that the data the Lancet paper presented from Mehra, Desai et al. were fabricated, for many statistical and logistical reasons that are discussed in the links I have provided, especially in that letter with, now, 182 signatories. Yet the Mehra, Desai et al. paper, in the world's top medical journal, was intended to be the death knell for hydroxychloroquine (and subsequently for many patients with Covid). Are you starting to see how deep the rot goes?
Understand that the WHO's initial plan for its Covid clinical trial had entirely omitted chloroquines. Then, at the last minute, it agreed to add in chloroquines. But now, it has deemed the drugs unsafe and pulled them from its multicenter trial, so we may never know how well they work to prevent and treat Covid-19.
This is bizarre, because the WHO's Essential Medicines list, which "contains the medications considered to be most effective and safe to meet the most important needs in a health system," includes chloroquine. So it's safe for malaria, but unsafe for Covid?
This is bizarre, because the WHO's Essential Medicines list, which "contains the medications considered to be most effective and safe to meet the most important needs in a health system," includes chloroquine. So it's safe for malaria, but unsafe for Covid?
Tedros Adhanom Ghebreyesus, the WHO's first non-physician Director General, said exactly that: the drug is safe, except if you want to use it for Covid:
"Tedros told patients taking the medication for its well-established uses beyond COVID-19 that they shouldn't worry. "This concern relates to the use of hydroxychloroquine and chloroquine in COVID-19," he said. "I wish to reiterate that this drug is accepted as generally safe for use in patients with autoimmune diseases and malaria."'
This is an oxymoron: an inherent contradiction. Either the drug is dangerous, or it isn't. Tedros' statement sounds like it was created by a PR firm. Is this why a non-physician was chosen, for the first time, to lead the WHO? So, lacking understanding, he could read these lines with a straight face?
Should we be surprised that WHO has stopped testing hydroxychloroquine without its Data Safety Monitoring Board (DSMB) issuing a warning? The DSMB is the official safety monitor for clinical trials. Strangely, it wasn't the DSMB which initiated this halt. It was the WHO's "steering committee," which met over the last weekend, and decided to stop the testing of hydroxychloroquine. I wonder if the trial protocol even allowed for that. Which steering committee, exactly, came up with this, and who empowered them to do so?
According to the WHO's chief scientist, Dr. Swaminathan, the WHO hasn't even seen any of its trial data indicating a problem with hydroxychloroquine. They had no data, but they had to meet on a weekend to stop testing hydroxychloroquine? How could Tedros possibly explain this?
"Tedros cited the British journal, The Lancet, which published findings Friday showing that hydroxychloroquine doesn't help COVID-19 patients and might even increase deaths."
So the sketchy Lancet study, based on a rough (no accounting for confounders) analysis from a databased owned by the person who analyzed it, who has himself refused to provide even the names of the hospitals from which the data came, was the basis for taking hydroxychloroquine out of the WHO trial. Interesting.
"The executive group has implemented a temporary pause of the hydroxychloroquine arm within the Solidarity Trial while the safety data is reviewed by the data safety monitoring board. The other arms of the trial are continuing,” Tedros said in an online briefing from Geneva."
WHO is wedded to Gates, GAVI (Global Vaccine Alliance formed by the Gates Foundation) and Big Pharma. Now that the US has pulled its funding for WHO, Bill Gates (who has invested billions in experimental Covid vaccines and claimed there will be no return to normal life until we have a vaccine) is WHO's largest donor. Did Bill Gates steer WHO's "steering committee" in the direction he wanted?
Or was this the WHO's shot at Trump for pulling about $450 million per year in funding? According to the May 27 South China Morning Post, "the World Health Organisation has launched a foundation to help it raise funds from individuals and companies, as the UN agency seeks to broaden its donor base." Is the new foundation the WHO's vehicle to more easily accept Pharma payback, including for its hydroxychloroquine decision?
-----------------
In light of the WHO decision, other large clinical trials of HCQ also went into emergency mode last weekend to decide whether to halt their HCQ arms. Here is what the 2 chief investigators of the UK's Recovery trial had to say:
‘The Committee found that the effects of hydroxychloroquine on mortality reported in the analysis by Mehra were not consistent with those observed in the RECOVERY trial. The Committee therefore recommended that the trial continue recruitment without interruption, a recommendation that was endorsed on Sunday by the MHRA.
-----------------
Sharyl Attkisson blew up the new NIH (NIAID) recommendations on Covid-19 treatment, which panned HCQ and promoted Remdesivir. Sharyl discovered that sixteen of the NIH's guideline authors (including 2 of 3 co-chairs) had existing or prior financial relationships with Gilead, the maker of Remdesivir, but none with hydroxychloroquine makers. How did such a Gilead-heavy panel come to be constituted? The members were appointed by the financially conflicted co-chairs.
------------------
Who chose to use HCQ? Trump used it for prevention. Virologist and SARS expert Ian Lipkin, MD, who was offered convalescent serum by the Chinese doctors he works with, refused it (despite its positive track record in Covid-19 therapy) and used HCQ instead. While he was initially quite ill, he got over his illness quickly.
Boris Johnson, Prince Charles, and other recovered luminaries have failed to report how they were treated, and the media didn't ask.
But now, based on WHO stopping its trial, allegedly on the basis of one very questionable paper in the Lancet, several countries have started banning the use of hydroxychloroquine by personal physicians.
-------------------
"Medical Journals Are an Extension of the Marketing Arm of Pharmaceutical Companies," wrote Richard Smith, former editor-in-chief of the British Medical Journal (BMJ), in 2005. He then cited several of his fellow editors at other journals: “Journals have devolved into information laundering operations for the pharmaceutical industry,” wrote Richard Horton, still editor-in-chief of the Lancet... Marcia Angell, former editor of the New England Journal of Medicine, lambasted the industry for becoming “primarily a marketing machine” and for Pharma co-opting “every institution that might stand in its way”... Jerry Kassirer, another former editor of the New England Journal of Medicine, argues that the industry has deflected the moral compasses of many physicians..." It is utterly demoralizing that Lancet editor Richard Horton, who denounced medical journals with the moniker "information laundering operations" complied with the publication of the questionable Lancet piece last week. Update: Horton now calls the article a "fabrication."
--------------------
Tedros claimed WHO just imposed a "temporary pause" on its HCQ trial, giving the data monitors the chance to carefully do their review. But we know from an exclusive piece by Reuters and another in the Indonesia Times that the WHO quickly directed Indonesia to stop using HCQ for all Covid treatment, not only in WHO-affiliated clinical trials, before such review has taken place. Did WHO send similar advice to the other 192 member nations, or perhaps only to those who were using HCQ widely and having good results? It appears WHO is trying to obliterate evidence that might support widespread use of hydroxychloroquine, and might disadvantage both Gilead's Remdesivir, and anticipated coronavirus vaccines, each to cost hundreds or thousands of times more than hydroxychloroquine (which is generic and costs less than $1.00 per course in some countries).
Didn't the recent NIH/NIAID treatment guidelines attempt to make doctors switch from using HCQ to using Remdesivir via a stacked deck of guideline authors who work or worked for Gilead?
Are we seeing a highly coordinated assault on unbiased scientific evidence and reporting by the WHO, the NIH, some national public health agencies, and medical journals and their authors?
Aren't we seeing a gargantuan assault on our right to be fairly informed and choose our own medical treatments? It appears that the premier agencies and organizations, whose ostensible mission is to safeguard our health, have been captured and redirected.
Yet again, it seems, the Covid crisis has exposed the unthinkable. We cannot expect the institutions of government and society to have our best interests at heart. They clearly don't.
We are in the midst of a crisis that requires specialized knowledge to understand and respond to. We have a federal government whose many agencies have spent over $100 billion dollars, since 9/11, on pandemic planning and response. Yet when the crisis came, they offered almost none of the masks and PPE they were supposed to have stockpiled. They had no tests, no drugs, no vaccines, and precious little good advice. Federal agencies interfered with attempts by the market to provide tests and drugs. We have learned, bitterly, it is every man/woman for him/herself.
Yet we can only change this system by working together. We have to figure out how to do so. Shining a light on the corrupt underbelly of our system is the first step. Let's go even further.
Update May 30: India and Indonesia say they stand by use of hydroxychloroquine, as they are convinced of its benefit, but Indonesia will comply with WHO guidance and cease using it in patients who enroll in the Solidarity trial. Turkey too.
Update May 31: The Scientist has uncovered a long litany of lies, exaggerations and shady businesses associated with Dr. Sapan Desai, MD, PhD, MBA in a detailed investigative piece here.
Didn't the recent NIH/NIAID treatment guidelines attempt to make doctors switch from using HCQ to using Remdesivir via a stacked deck of guideline authors who work or worked for Gilead?
Are we seeing a highly coordinated assault on unbiased scientific evidence and reporting by the WHO, the NIH, some national public health agencies, and medical journals and their authors?
Aren't we seeing a gargantuan assault on our right to be fairly informed and choose our own medical treatments? It appears that the premier agencies and organizations, whose ostensible mission is to safeguard our health, have been captured and redirected.
Yet again, it seems, the Covid crisis has exposed the unthinkable. We cannot expect the institutions of government and society to have our best interests at heart. They clearly don't.
We are in the midst of a crisis that requires specialized knowledge to understand and respond to. We have a federal government whose many agencies have spent over $100 billion dollars, since 9/11, on pandemic planning and response. Yet when the crisis came, they offered almost none of the masks and PPE they were supposed to have stockpiled. They had no tests, no drugs, no vaccines, and precious little good advice. Federal agencies interfered with attempts by the market to provide tests and drugs. We have learned, bitterly, it is every man/woman for him/herself.
Yet we can only change this system by working together. We have to figure out how to do so. Shining a light on the corrupt underbelly of our system is the first step. Let's go even further.
Update May 30: India and Indonesia say they stand by use of hydroxychloroquine, as they are convinced of its benefit, but Indonesia will comply with WHO guidance and cease using it in patients who enroll in the Solidarity trial. Turkey too.
Update May 31: The Scientist has uncovered a long litany of lies, exaggerations and shady businesses associated with Dr. Sapan Desai, MD, PhD, MBA in a detailed investigative piece here.
Sunday, May 24, 2020
India's NIH (the ICMR) expands the use of hydroxychloroquine to all frontline health workers, police, etc.
I hope you have noticed that while the mass media have spent 2 months railing about the dangers of hydroxychloroquine and how it may kill you, they have had a hard time finding doctors to issue the alarm on-camera. Practicing doctors usually know that the risks of HCQ are no greater than the risk of other drugs they prescribe routinely, making it hard to utter dire warnings.
And now, India's official medical research arm has reported on 3 studies of HCQ prophylaxis it conducted. They think the drug is safe enough to use for prevention, and they think it works. So, the ICMR has expanded its recommendations for hydroxychloroquine as a Covid preventive: now recommending it for all first responders and medical personnel, unless there is a medical contraindication:
"The Indian Council of Medical Research (ICMR) has issued revised guidelines for use of hydroxychloroquine (HCQ), the malaria drug, as a preventive medication for asymptomatic healthcare workers in non-Covid-19 hospitals, frontline staff on surveillance duty in containment zones and paramilitary/police personnel involved in coronavirus infection related activities.
"The Joint Monitoring Group and the NTF have recommended prophylactic use of HCQ in asymptomatic frontline workers, such as surveillance workers deployed in containment zones and paramilitary/police personnel involved in Covid-19 related activities, asymptomatic household contacts of laboratory confirmed cases and all asymptomatic healthcare workers involved in containment and treatment of Covid-19 and working in non-Covid hospitals/non-Covid areas of Covid hospitals/blocks," the ICMR said, here on Saturday (23 May)."
The recommendation was made after the Joint Monitoring Group under the Chairmanship of Directorate General of Health Services (DGHS) and including representatives from AIIMS, ICMR, National Centre for Disease Control, National Disaster Management Authority, WHO and experts drawn from central government hospitals reviewed the prophylactic use of hydroxychloroquine (HCQ) in the context of expanding it to healthcare and other frontline workers deployed in non-COVID-19 and COVID-19 areas.
Three new categories - all asymptomatic healthcare workers working in non-COVID hospitals/areas of COVID hospitals/blocks, asymptomatic frontline workers such as surveillance workers deployed in containment zones and paramilitary/police personnel involved in COVID-19 related activities - have now been included.
According to the revised advisory, "at NIV, Pune, the report of the in-vitro testing of HCQ for antiviral efficacy showed reduction of infectivity and log reduction in viral RNA copy of SARs-CoV2".
"The drug is contraindicated in persons with known case of retinopathy, hypersensitivity to HCQ and cardiac rhythm disorders," it said.
----------
Dr. Peter Breggin, psychiatrist and author, saved me a lot of work by compiling additional information on the safety and efficacy of hydroxychloroquine. His article can be read here. Is the purpose of the media warnings to keep us scared out of our minds about this virus, which may actually be quite treatable? And preventable?
I am now taking vitamin C, vitamin D and zinc, and will be adding hydroxychloroquine at the first sign of a virus. I will continue to be careful, but with convincing reports that 90% of cases are asymptomatic (and presumably, though not certainly, will become immune with no illness at all) I am finished being scared.
My interview with Dr. Joseph Mercola on the origin of Covid-19
STORY AT-A-GLANCE
· The manufactured anthrax crisis of 2001 initiated the PATRIOT Act, one of the most severe compromises of our personal freedoms up to that point. Now, the COVID-19 pandemic is being used to take away even more freedoms
· It appears influential virologists are protecting the narrative that SARS-CoV-2 arose naturally, and did not originate from a lab in China or elsewhere, even though their scientific justification for that conclusion is faulty
· Strong evidence suggests SARS-CoV-2 cannot be the result of a natural mutation
· The National Institutes of Allergy and Infectious Diseases (NIAID), under Dr. Anthony Fauci’s leadership, has funded gain-of-function research on coronaviruses for about two decades
· Efforts to develop coronavirus vaccines have failed for two decades, as the vaccines tend to cause paradoxical immune enhancement resulting in damaging and lethal cytokine storms
Click on the link for the rest.
Saturday, May 23, 2020
A Lancet study with over-the-top data tries to sound a death knell for chloroquine, fails
A big news story came out today regarding the results of a Lancet study of chloroquine, hydroxychloroquine and azithromycin in hospitalized Covid-19 patients. The first author is, naturally, from Harvard.
What the authors found were considerably higher rates of arrhythmia and death in the patients who received a chloroquine drug, with even worse outcomes if patients received azithromycin (Z-pak) too.
Important, smart doctors were interviewed, and they said things like, "Now we know these drugs kill." "Stop using them, except possibly in a clinical trial setting."
Maybe the Lancet study is giving us the last word on the chloroquine drugs and Covid-19.
But let me tell you a few things about this study that give me pause.
The retrospective study included 96,000 people, of whom nearly 15,000 received a chloroquine drug. Now those are really large numbers, so this should be a well powered study. How did the authors get so much data? The second author, Sapan S Desai (SSD), founder of Surgisphere Corporation, appears to have provided it. Which makes me wonder how the 671 hospitals or the 96,000 patients felt about their medical and financial data being used, with no ethical review...
Besides the privacy issues (having a private US company get hold of blended medical and financial records from 671 hospitals on 6 continents) is the issue of the accuracy of this data and analysis. Could the data have been manipulated? I doubt data accuracy was checked with 671 individual hospitals...who might not have been happy their data were being used... How do you verify the validity of data from so many different sites?
In the UK, 33% of 17,000 hospitalized patients died from Covid-19. A Chinese study found 28% of those hospitalized died. A US study revealed a 21% mortality rate in those hospitalized for Covid-19. Another US study had a 20.3% mortality in hospitalized patients, but found that those who received chloroquine drugs were sicker than those who did not.
Yet in the Surgisphere Corporation dataset of 96,000 hospitalized patients, only 11.1% of hospitalized patients died. And in the control group, who did not get any chloroquine drugs, in-hospital mortality was only 9.3%. Mortality in the chloroquine groups ranged from 18% to 23.8%.
I find the data presented in this Lancet article hard to believe. The mortality rates in the non-chloroquine patients are simply too good to be true. It is also possible that the chloroquine patients were a sicker cohort. In any event, their mortality rates are in keeping with overall rates in the US, and are better than published rates in the UK and China.
How did this group of hospitals do twice as well as the US, and 3 times as well as the UK in preventing Covid deaths???
I don't think the debate on use of these drugs is over.
Update May 31: The Scientist has uncovered a long litany of lies and exaggerations from Dr. Sapan Desai in a detailed investigative piece here. And I uncovered the odd fact that Dr. Desai wrote a 2013 article titled "Combating fraud in medical research."
What the authors found were considerably higher rates of arrhythmia and death in the patients who received a chloroquine drug, with even worse outcomes if patients received azithromycin (Z-pak) too.
Important, smart doctors were interviewed, and they said things like, "Now we know these drugs kill." "Stop using them, except possibly in a clinical trial setting."
Maybe the Lancet study is giving us the last word on the chloroquine drugs and Covid-19.
But let me tell you a few things about this study that give me pause.
The retrospective study included 96,000 people, of whom nearly 15,000 received a chloroquine drug. Now those are really large numbers, so this should be a well powered study. How did the authors get so much data? The second author, Sapan S Desai (SSD), founder of Surgisphere Corporation, appears to have provided it. Which makes me wonder how the 671 hospitals or the 96,000 patients felt about their medical and financial data being used, with no ethical review...
"Acquisition of data and statistical analysis of the data were supervised and performed by SSD...
SSD is the founder of Surgisphere Corporation.
The Surgical Outcomes Collaborative (Surgisphere Corporation, Chicago, IL, USA) consists of de-identified data obtained by automated data extraction from inpatient and outpatient electronic health records, supply chain databases, and financial records. The registry uses a cloud-based health-care data analytics platform that includes specific modules for data acquisition, data warehousing, data analytics, and data reporting."The data came from 671 hospitals on 6 continents. Wow. And here is just a bit of what the authors tell us about data collection:
"The standardised Health Level Seven-compliant data dictionary used by the Collaborative serves as the focal point for all data acquisition and warehousing. Once this data dictionary is harmonised with electronic health record data, data acquisition is completed using automated interfaces to expedite data transfer and improve data integrity. Collection of a 100% sample from each health- care entity is validated against financial records and external databases to minimise selection bias. To reduce the risk of inadvertent protected health information disclosures, all such information is stripped before storage in the cloud-based data warehouse...The data collection and analyses are deemed exempt from ethics review."You harmonise the data, then you improve its integrity. Wait, what? The only way to improve the integrity of electronic data is to compare it to hard copies of the data. I am guessing that what the authors mean is that data points that an algorithm determined were incorrect got changed or dropped. And financial records were available, too. How the heck did that happen? Then Surgisphere stored all of this in the cloud, after de-identifying it.
Besides the privacy issues (having a private US company get hold of blended medical and financial records from 671 hospitals on 6 continents) is the issue of the accuracy of this data and analysis. Could the data have been manipulated? I doubt data accuracy was checked with 671 individual hospitals...who might not have been happy their data were being used... How do you verify the validity of data from so many different sites?
In the UK, 33% of 17,000 hospitalized patients died from Covid-19. A Chinese study found 28% of those hospitalized died. A US study revealed a 21% mortality rate in those hospitalized for Covid-19. Another US study had a 20.3% mortality in hospitalized patients, but found that those who received chloroquine drugs were sicker than those who did not.
Yet in the Surgisphere Corporation dataset of 96,000 hospitalized patients, only 11.1% of hospitalized patients died. And in the control group, who did not get any chloroquine drugs, in-hospital mortality was only 9.3%. Mortality in the chloroquine groups ranged from 18% to 23.8%.
I find the data presented in this Lancet article hard to believe. The mortality rates in the non-chloroquine patients are simply too good to be true. It is also possible that the chloroquine patients were a sicker cohort. In any event, their mortality rates are in keeping with overall rates in the US, and are better than published rates in the UK and China.
How did this group of hospitals do twice as well as the US, and 3 times as well as the UK in preventing Covid deaths???
I don't think the debate on use of these drugs is over.
Update May 31: The Scientist has uncovered a long litany of lies and exaggerations from Dr. Sapan Desai in a detailed investigative piece here. And I uncovered the odd fact that Dr. Desai wrote a 2013 article titled "Combating fraud in medical research."
Tuesday, May 19, 2020
CDC: Chloroquine works against SARS: 2005 International Virology Journal
US CDC proved efficacy of chloroquine for SARS 15 years ago.
Virol J. 2005; 2: 69.
Chloroquine is a Potent Inhibitor of SARS Coronavirus Infection and Spread
Published online 2005 Aug 22. doi: 10.1186/1743-422X-2-69
1Special Pathogens Brach, Division of Viral and Rickettsial Diseases, Centers for Disease Control and Prevention, 1600 Clifton Road, Atlanta, Georgia, 30333, USA
2Laboratory of Biochemical Neuroendocrinology, Clinical Research Institute of Montreal, 110 Pine Ave West, Montreal, QCH2W1R7, Canada
 Corresponding author.
Corresponding author.
Martin J Vincent: vog.cdc@tnecnivm; Eric Bergeron: ac.cq.mcri@eregreb; Suzanne Benjannet: ac.cq.mcri@snajneb; Bobbie R Erickson: vog.cdc@1noskcirEB; Pierre E Rollin: vog.cdc@nilloRP; Thomas G Ksiazek: vog.cdc@kezaisKT; Nabil G Seidah: ac.cq.mcri@nhadies; Stuart T Nichol: vog.cdc@lohciNS
Abstract
Background
Severe acute respiratory syndrome (SARS) is caused by a newly discovered coronavirus (SARS-CoV). No effective prophylactic or post-exposure therapy is currently available.
Results
We report, however, that chloroquine has strong antiviral effects on SARS-CoV infection of primate cells. These inhibitory effects are observed when the cells are treated with the drug either before or after exposure to the virus, suggesting both prophylactic and therapeutic advantage. In addition to the well-known functions of chloroquine such as elevations of endosomal pH, the drug appears to interfere with terminal glycosylation of the cellular receptor, angiotensin-converting enzyme 2. This may negatively influence the virus-receptor binding and abrogate the infection, with further ramifications by the elevation of vesicular pH, resulting in the inhibition of infection and spread of SARS CoV at clinically admissible concentrations.
Conclusion
Chloroquine is effective in preventing the spread of SARS CoV in cell culture. Favorable inhibition of virus spread was observed when the cells were either treated with chloroquine prior to or after SARS CoV infection. In addition, the indirect immunofluorescence assay described herein represents a simple and rapid method for screening SARS-CoV antiviral compounds.
This article has been cited by other articles in PMC.
Subscribe to:
Posts (Atom)









