From the beginning, the official COVID-19 narrative has been inconsistent, hypocritical and/or contradictory because medical authorities used double standards to create the illusion their narrative was logical and sensible.
We are not only in an epidemiological crisis, we also are in an epistemological crisis. How do we know what we know? What differentiates opinion from a justified belief?
For nearly two years, the public has been inundated by a sophisticated messaging campaign that urges us to “trust the science.”
But how can a non-scientist know what the science is really saying?
Legacy media sources offer us an easy solution: “Trust us.”
Legions of so-called “independent” fact-checking sites that serve to eliminate any wayward thinking keep those with a modicum of skepticism in line.
“Research” has been redefined to mean browsing Wikipedia citations.
Rather than being considered for their merit, dissenting opinions are more easily dismissed as misinformation by labeling their source as untrustworthy.
How do we know these sources are untrustworthy? They must be if they offer a dissenting opinion!
This form of circular reasoning is the central axiom of all dogmatic systems of thought. Breaking the spell of dogmatic thinking is not easy, but it is possible.
In this article I describe six examples of double standards medical authorities have used to create the illusion their COVID-19 narrative is logical and sensible.
This illusion has been used with devastating effect to raise vaccine compliance.
Rather than citing scientific publications or expert opinions that conflict with our medical authorities’ narrative — information that will be categorically dismissed because it appears on The Defender — I will instead demonstrate how, from the beginning, the official narrative has been inconsistent, hypocritical and/or contradictory.
1. COVID deaths are ‘presumed,’ but vaccine deaths must be ‘proven’
As of April 8, VAERS included 26,699 reports of deaths following COVID vaccines.
The Centers for Disease Control and Prevention (CDC) officially acknowledges only nine of these.
In order to establish causality, the CDC requires autopsies to rule out any possible etiology of death before the agency will place culpability on the vaccine.
But the CDC uses a very different standard when it comes to identifying people who died from COVID.
The 986,000 COVID deaths reported by the CDC here are, as footnote [1] indicates, “Deaths with confirmed or presumed [emphasis added] COVID-19.”
If a person dies with a positive PCR test or is presumed to have COVID, the CDC will count that as COVID-19 death.
Note that in the CDC’s definition, a COVID fatality does not mean the person died from the disease, only with the disease.
Why is an autopsy required to establish a COVID vaccine death but not to establish a COVID death?
Conversely, why is recent exposure to SARS-CoV-2 prior to a death sufficient to establish causality — but recent exposure to a vaccine considered coincidental?
2. CDC uses VAERS data to investigate myocarditis yet claims VAERS data on vaccine deaths is unreliable
On June 23, 2021, the CDC’s Advisory Committee on Immunization Practices met to assess the risk of peri/myocarditis following COVID vaccination, especially in young males.
This was the key slide in this presentation:
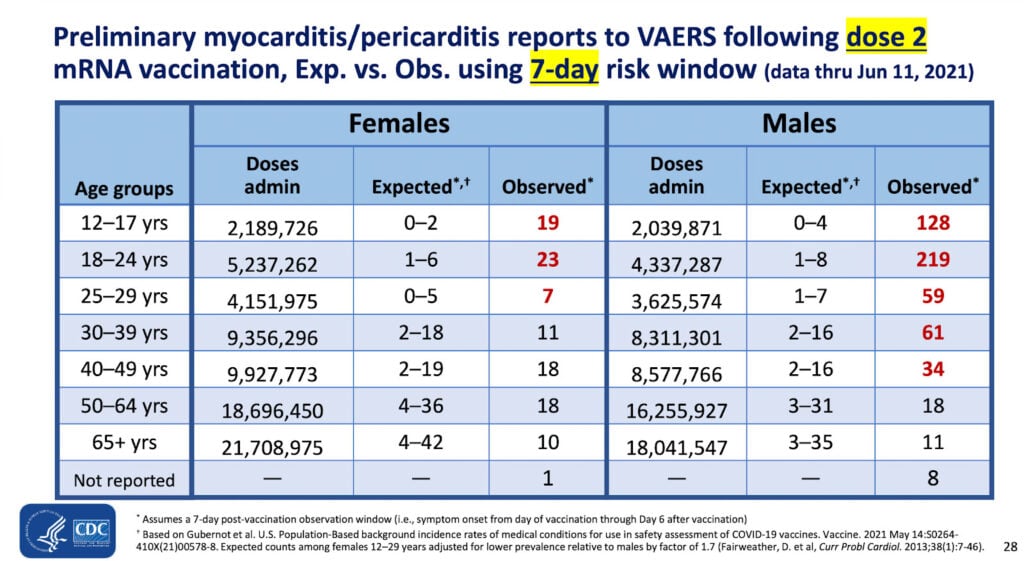
The observed risk of myocarditis is 219 in about 4.3 million second doses of COVID vaccine in males 18 to 24 years old.
The CDC is fine with using VAERS data to assess risk of myocarditis following vaccination — yet the agency rejects all but nine of the 26,699 reports of deaths following the vaccines.
Why does the CDC trust the peri/myocarditis data in VAERS but not the data on deaths?
One reason may be because the onset of myocarditis symptoms is closely tied to the time of vaccination.
In other words, because this condition closely follows inoculation the two events are highly correlated and suggestive of causation.
For example, here is another slide from the same presentation:
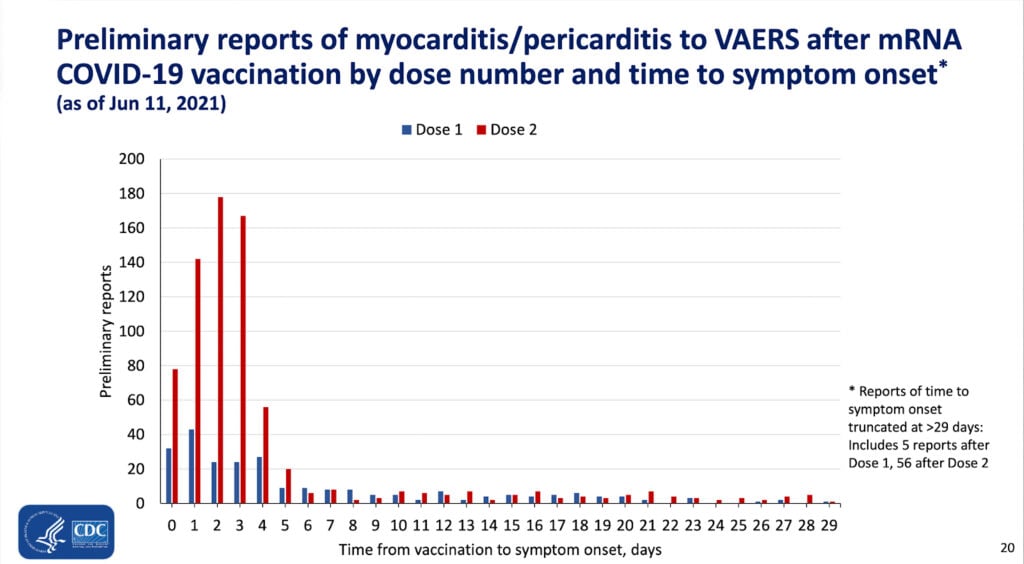
The majority of cases of vaccine-induced peri/myocarditis suffered symptoms within the first few days after injection. As explained above, this is highly suggestive of a causative effect of the vaccine.
A recent study in The Lancet included a similar graph, taken directly from VAERS, on deaths following vaccination:
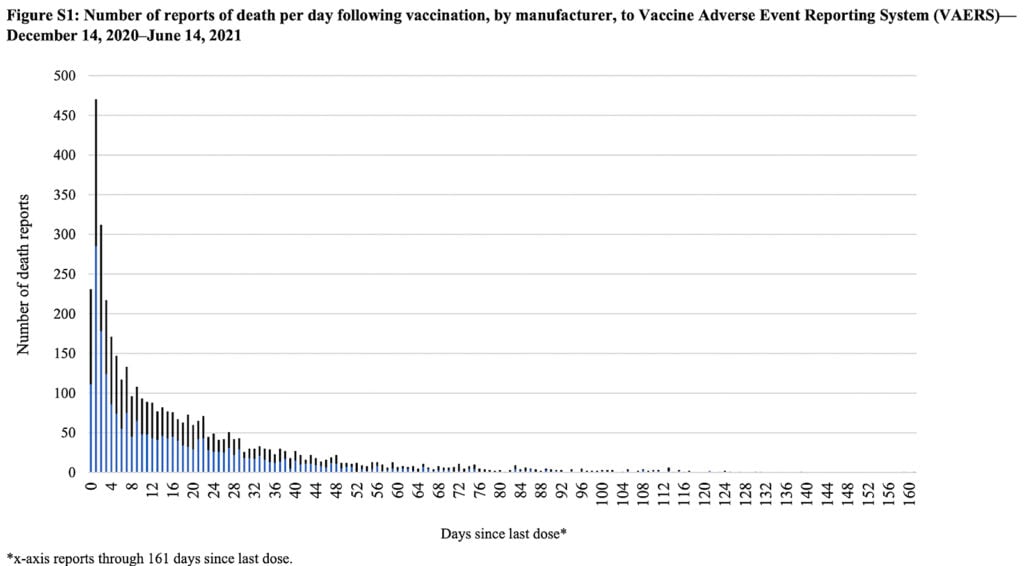
Once again, the event (death) closely follows vaccination in the majority of cases.
As we regard the two graphs above we should acknowledge that the temporal relationship between the injection and the adverse event is suggestive of causation but does not stand as proof of such.
However, it is also important to note that if the vaccination caused the deaths, that is exactly what the plot would look like.
It should be clear that the CDC has no justification for dismissing VAERS deaths if the agency is willing to accept reports of myo/pericarditis from the very same reporting system.
3. CDC pushes ‘relative risk’ for determining vaccine efficacy, but uses ‘absolute risk’ to downplay risk of adverse events
In Pfizer’s Phase 3 trial, nine times more placebo recipients developed severe COVID than those vaccinated during the short period of observation. This constitutes a relative risk reduction of 90%.
This seemed an encouraging finding and was used as a major talking point to compel the public to accept this experimental therapy despite the absence of any long-term data.
However, the risk of a trial participant contracting severe COVID (Table S5) was 1 in 21,314 (0.0047%) if they were vaccinated.
If they received the placebo, the risk was still only 9 in 21,259 (0.0423%).
The vaccine reduced the absolute risk of contracting severe disease by 0.038%.
Mainstream media and the CDC never mentioned the minuscule reduction in absolute risk of contracting severe COVID by getting inoculated.
Moreover, with 0.6% of vaccine recipients in the trial suffering a serious vaccine injury (one that results in death, medical or surgical intervention, hospitalization or an impending threat to life), approximately 16 serious adverse events will result for every serious case of COVID prevented by vaccination.
However, when it comes to risk of myo/pericarditis, the CDC states, “Myocarditis and pericarditis have rarely been reported, especially in adolescents and young adult males within several days after COVID-19 vaccination.”
The CDC further states, “While absolute risk remains small, the risk for myocarditis is higher for males ages 12 to 39 years…”
In other words, the risk of adverse events is being considered in absolute terms, not relative.
The CDC presentation slide above (Table 1) indicates the relative risk of contracting myo/pericarditis in males 18 to 24 is 27 to more than 200 times higher than expected in (unvaccinated) young men that age.
When assuaging the public’s fear around vaccine-induced myocarditis, the CDC finds it useful to cite absolute risk — yet when promoting the efficacy of the vaccine, the CDC emphasizes relative risks.
This double standard has been quietly and masterfully employed to reduce vaccine hesitancy and encourage compliance.
4. FDA requires randomized control studies for early treatment medications — but not for boosters
The CDC reports that as of April 8, 98.3 million Americans had received a COVID booster.
On March 29, the U.S. Food and Drug Administration (FDA) authorized a second booster for the immunocompromised and adults over age 50.
These authorizations were made not because of solid evidence the boosters are effective but rather to remedy the fact that the primary vaccine series has been widely shown to have waning efficacy within a few months.
As reported by The Defender, Dr. Peter Marks, director of the FDA’s vaccine division, Center for Biologics Evaluation and Research, admitted the fourth booster dose approved last week was a “stopgap measure” — in other words, a temporary measure to be implemented until a proper solution may be found in the future.
Despite the lack of solid evidence, the FDA continues to recommend and authorize boosters.
Yet when it comes to early treatment options, the agency holds medicines — including those the agency has already licensed and approved for other uses — to a different standard.
In this CNN interview from August 2021, Dr. Anthony Fauci, head of the National Institute of Allergy and Infectious Diseases, warns people not to take ivermectin for COVID because “there is no clinical evidence that this works.”
With regard to hydroxychloroquine, Fauci said, “We know that every single good study — and by good study, I mean randomized control study in which the data are firm and believable — has s shown that hydroxychloroquine is not effective in the treatment of Covid-19”, as reported by the BBC on July 29, 2020.
Where, then, are the randomized control studies in which the data are firm and believable that show boosters are effective at preventing COVID?
There aren’t any. None have been done.
As of today, the FDA still refuses to authorize the use of ivermectin and hydroxychloroquine to treat COVID despite hundreds of studies that demonstrate significant benefits (ivermectin, hydroxychloroquine) in prevention as well as early and late treatment.
The double standard here is blatant. There are no randomized control studies that show boosters are effective in preventing COVID.
Nevertheless, these experimental therapies have the FDA’s blessing while inexpensive, highly effective safe and proven medicines are ignored despite the enormous evidence that supports their use.
5. FDA uses immunobridging to justify Pfizer shots for young kids, but rejects antibodies as indicative of immune protection from COVID
Immunobridging is a method of inferring a vaccine’s effectiveness in preventing disease by assessing its ability to elicit an immune response through the measurement of biochemical markers, typically antibody levels.
The FDA asserts the presence of SARS-COV-2 antibodies is not necessarily indicative of immune protection from COVID.
Moreover, the FDA’s Vaccine and Related Biologics Product Advisory Committee reached a consensus last week that antibody levels cannot be used as a correlate for vaccine effectiveness.
Their decision is consistent with the CDC’s executive summary of a science brief released on October 29, 2021:
“Data are presently insufficient to determine an antibody titer threshold that indicates when an individual is protected from infection.”
Nevertheless, the FDA used immunobridging as a means to justify authorization of the Pfizervaccine to children ages 5 to 11, as explained in The Defender here and here.
Because there were no deaths or serious cases of COVID in the pediatric trial, the FDA chose to reject its own position (and that of its advisory committee) regarding antibody titers as a correlate for vaccine efficacy.
6. Causation must be proven for vaccine injuries, but correlation suffices for proving vaccine efficacy
When it comes to vaccine injuries the public is often reminded that correlation does not equal causation.
In other words, just because an injury was preceded by inoculation doesn’t mean the vaccine caused the injury.
But what constitutes causation in medicine? A mechanism of action needs to be identified and pathological studies must confirm this mechanism while eliminating other potential causative factors. Causation can be proven only on a case-by-case basis.
Proving causation requires an enormous burden of proof in medicine.
For example, does smoking cause lung cancer? The answer is yes, it can. That doesn’t mean that it will.
However, when it comes to the benefit of medical intervention, such as a vaccine, causation does not have to be established. Correlation suffices.
In the COVID vaccine trials, fewer vaccinated people contracted COVID than unvaccinated ones. Yet there were those who received the vaccine who contracted the disease anyway.
To be fair, this is how all new medical interventions are evaluated. The benefit doesn’t have to be caused by the vaccine in the strictest sense, there just has to be a correlation between vaccination and a relative protective effect.
The more often this happens, the more confident we can be that the outcome wasn’t simply a coincidence.
Likewise, when it comes to assessing the harm of medical intervention, the most sensible outcome to consider is mortality. After all, what would be the point of introducing a vaccine that prevented some deaths while causing more?
Nevertheless, this is, in fact, what we have done with the Pfizer product. The interim results from the Phase 3 trial demonstrated that all-cause mortality in the vaccinated cohort was higher than in the placebo.
This glaring problem gets brushed aside because there were two deaths from COVID in the placebo arm versus just one in the vaccinated cohort, allowing the vaccine manufacturer to claim a 50% efficacy in preventing this outcome.
However, if we attribute a protective benefit to the vaccine in preventing this one fatality, we must also conclude that the vaccine was responsible for the extra death when considering mortality from all causes.
Doing otherwise would be applying yet another double standard.
How the pandemic could have played out differently
To summarize how devastating the use of these double standards in crafting the “safe and effective” narrative was, let’s look at how different the situation would be if we had adopted the opposite standard:
- There would have been an extremely low number of deaths from COVID. Very few, if any, autopsies have definitively confirmed that a fatality was caused by SARS-CoV-2. If confirmation by autopsy is the standard, there have been essentially zero deaths from COVID during the pandemic.
On the other hand, if we presume the deaths registered in VAERS are in fact vaccine-induced fatalities — similar to how the CDC presumed many deaths from COVID — we can affirm there have been more than 26,000 vaccine deaths. - Using absolute risk reduction as a measure of efficacy, vaccines would have been widely rejected as ineffective, providing only a 0.038% risk reduction for contracting severe COVID.
- Ivermectin and hydroxychloroquine would have been readily available for people who got COVID. And for those who got the vaccine but got COVID anyway, these medicines would have been a great alternative to boosters, which wouldn’t have been approved due to the lack of a single randomized control study proving they work.
- No children between the ages of 5 and 11 would have received this risky, experimental vaccine as it wouldn’t have been authorized for this age group — because Pfizer’s pediatric trials did not demonstrate any meaningful outcomes in children ages 5 to 11.
- The Pfizer vaccine would no longer be in use because interim data demonstrated that all-cause mortality is higher in the vaccinated.
 0
0


















