What is a monoclonal antibody? In this case, it is a genetically engineered antibody produced in hamster ovary cells. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761328s000lbl.pdf
What are potential risks? According to the label, the only risks specified are rashes and anaphylaxis. This is a very uninformative label. Hardly worth the paper it is printed on.
Has any monoclonal antibody product been given on a mass scale to children ever? No
Has one ever been approved for newborns? Absolutely not.
Is it safe? The label waffles around the issue of safety. The hardest evidence for safety would be if fewer babies died overall in the group who received the RSV monoclonal than in the placebo group that got nothing. But in this case, the FDA briefer said there was an ‘imbalance’ of deaths in the two groups. The members knew what that meant, but I am sure the briefer hoped the audience did not. FDA ‘judged’ [i.e., guessed] that the excess deaths were not due to the monoclonal antibody. But how would FDA know that? You find out what the side effects are by doing clinical trials. And the label does not provide any details about side effects. It can be difficult to tell in a newborn. And the manufacturer and FDA appear to use that as a benefit, requiring less safety evaluation. The label does not inform us of the causes of deaths. This is extremely worrisome.
Will it work in the real world? There is already resistance in RSV strains to this product, and so as it gets used, the resistant strains will outperform the susceptible ones, and the RSV ecology is likely to change…meaning its use will likely not last very long.
Is this a drug or a vaccine? CDC is playing fast and loose with the definition of vaccine again, calling it a vaccine when convenient and a drug when that is more convenient. In order to get it onto the childhood vaccine schedule it becomes a vaccine—giving the manufacturer a waiver of liability. But it must be coded for reimbursement as a drug. It will be covered by the Affordable Care Act insurance program as a vaccine. But if there is an adverse event when it is used alone, the adverse event report will be filed in the drugs system (FAERS) belonging to FDA. If it is administered along with vaccines, the adverse event report will be sent to VAERS, belonging jointly to FDA and CDC.
What about interactions with other vaccines or drugs used at the same time? We have no idea, but just go ahead and mix and match ad lib, per FDA and CDC.
How much does it cost? Price to the CDC is $395 pp for 0-8 month olds, and $495 to other buyers. Price for 8-19 month olds is double that.
It will be recommended for virtually all babies, except those born in April, due to the way its supposed 5 months of efficacy is intended to overlap with the RSV fall-winter seasons.
MORE babies DIED who were TREATED than were in the control group. Same story as Pfizer's initial COVID shot trial. This will almost certainly kill many babies.
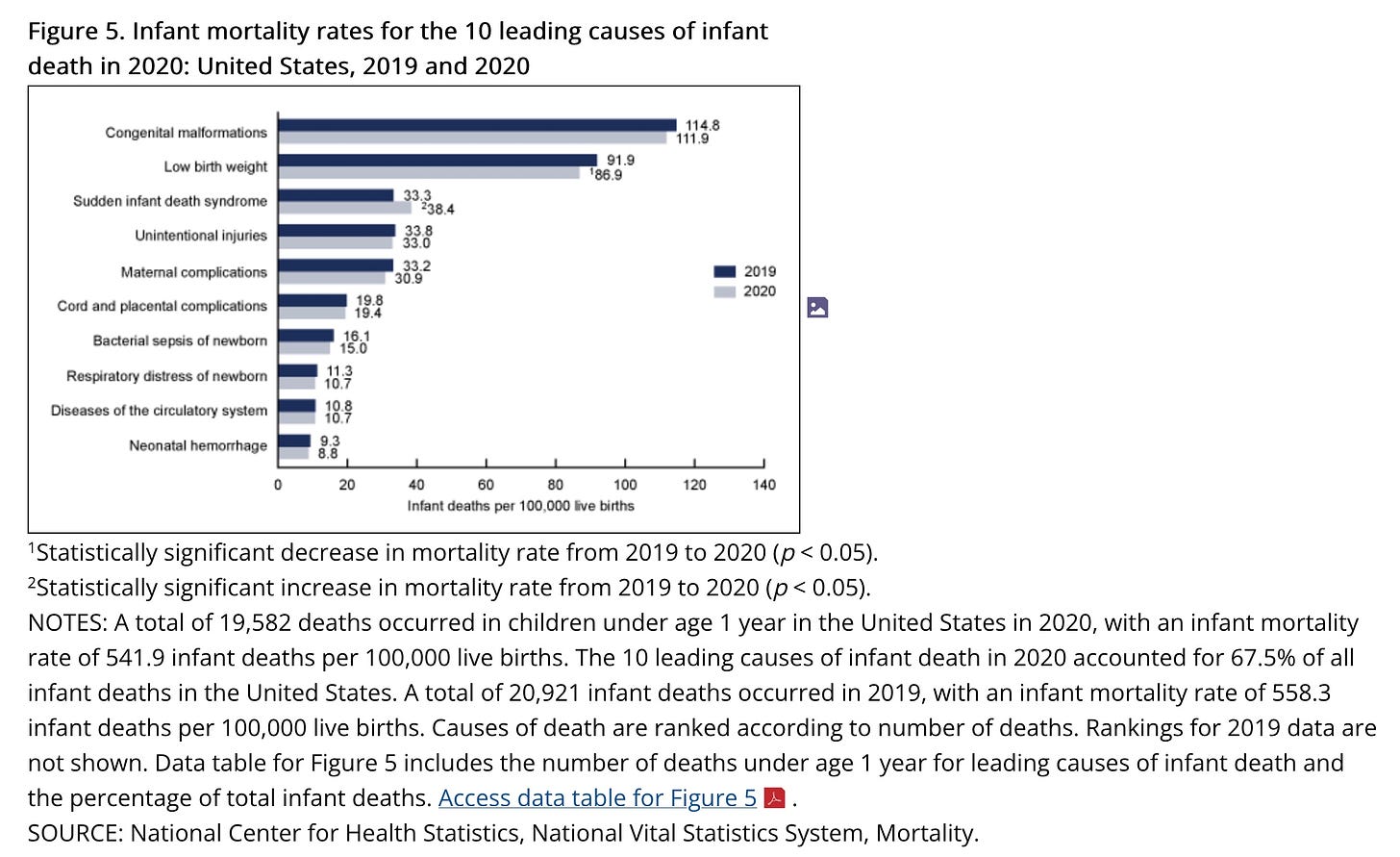
I previously revealed a published CDC paper from 2021 that showed only 25 babies up to one year of age die from RSV yearly in the entire US, averaged over 12 years. This is death certificate data, which CDC collects. It is considered THE GOLD STANDARD.
4 million babies are born yearly in the US. 20,000 die in their first year. RSV kills 0.125% of them. It is way down the list of top causes of death.
https://www.cdc.gov/nchs/products/databriefs/db427.htm#section_5
RSV almost never causes chronic problems, except perhaps asthma. Or maybe children with an asthma tendency are also more susceptible to severe RSV.
RSV does hospitalize a lot of US infants. It frightens parents and causes a lot of work for doctors. And so this group of pediatricians on CDC’s advisory committee went gaga over this new product, which is supposed to be 70-80% effective at preventing severe RSV disease.
Now let’s step back.
Here is what the Cleveland Clinic says about risks from other monoclonal antibodies:
https://my.clevelandclinic.org/health/treatments/22246-monoclonal-antibodies





No comments:
Post a Comment