The most recent summary on the D/P/T vaccine was published by CDC
in the MMWR of April 2018:
There are graphs in the MMWR article showing how cases of
all three diseases covered by the vaccine have changed over time.
These graphs show that there are almost no tetanus or
diphtheria cases in the US now. There
were 2 diphtheria cases reported in the entire US between 2004 and 2016.* While
diphtheria can theoretically spread between people, it does not seem to do so
in the US, presumably due to adequate herd immunity.
Tetanus is caused by bacteria that infect a wound or
incision, form an abscess and produce a toxin that is a nerve poison. Tetanus is a potentially life-threatening
illness, but can usually be prevented by proper care of wounds. There are about 30 cases and 1-6 deaths/year
in the US from tetanus.* It does not
spread from person to person.
Pertussis is a disease that looked like it, too was going to
be nearly wiped out during the 1980s.
But the pertussis component of the (whole cell) DPT vaccine being used
in those days was very toxic, causing many serious reactions and some deaths in
infants and children. So a newer, less
toxic vaccine was introduced, DTaP. This
reduced the number of serious reactions, but the new vaccine was later discovered
to be less effective at preventing pertussis than the old one. Per CDC:
"...reported rates of pertussis have
been observed to be significantly lower among children who had started their
vaccination series with DTP than among those who had started with DTaP (151,152)."
In the approximately
23 years since its US introduction, pertussis cases have risen dramatically. CDC says,
A growing body of evidence strongly suggests
that the change in vaccines in the late 1990s from whole-cell pertussis
vaccines to acellular pertussis vaccines in the childhood vaccine series has
caused the age-specific increases in pertussis incidence among children aged
7–10 years observed in the mid-2000s because of waning immunity (Figure 2) (28–30).
The CDC
decided to add another dose (the 6th dose) of a different DTaP vaccine (Tdap)
at age 12, and uptake of
this additional dose is at desired levels:
Since the introduction of Tdap in 2005,
coverage with Tdap in adolescents aged 13–17 years has increased substantially,
from 10.8% in 2006 to 86.4% in 2015 (33,34). Tdap coverage among adolescents
has met the Healthy People 2020 target
of 80% (35).
But Tdap
vaccine did not provide the immunity boost CDC desired. For the first
year after a Tdap vaccination, effectiveness against pertussis is estimated
about 67%, but by 4 years later effectiveness was only 9%. The
WHO says,
The disease,
caused by the bacterium Bordetella pertussis, is endemic in all countries.
Epidemic cycles have been occurring every 2 to 5 years (typically 3 to 4
years), even after the introduction of effective vaccination programmes and the
achievement of high vaccination coverage.
Most children who develop pertussis (80% in a Kaiser study) are fully vaccinated. Herd immunity cannot be achieved due to poor effectiveness of current vaccines, even if 100% of the population is fully vaccinated.
Conclusion:
Overall, children who lack DTap and Tdap vaccines pose no appreciable additional risk of infection to other children. This is because there is virtually no risk of contracting diphtheria in the US, there is no person-to-person spread of tetanus, and current pertussis vaccines provide limited immunity but also the likelihood of enhanced spread. These two effects likely balance out the transitory benefit of pertussis vaccination.
Meryl Nass, MD
*Centers for Disease Control and Prevention, Epidemiology
and Prevention of Vaccine-Preventable Diseases, 13th Edition. Appendix E.
Babies get much higher doses of
Diphtheria, Tetanus
and Pertussis
toxins in vaccines
than adults!
Tdap
|
DTaP
|
|||
Adults,
Pregnant Women,
12 year olds
|
2, 4, 6 months
18 months
5 years
|
|||
Boostrix
|
Adacel
|
Infanrix
|
Daptacel
|
|
Tetanus Toxoid
|
5 Lf *
|
5 Lf
|
10
Lf
|
5 Lf
|
Diptheria Toxoid
|
2.5 Lf
|
2 Lf
|
25
Lf
|
15
Lf
|
Pertussis Toxin
|
8 mcg
|
2.5 mcg
|
25
mcg
|
10 mcg
|
FHA
|
8 mcg
|
5 mcg
|
25
mcg
|
5 mcg
|
Pertactin
|
2.5 mcg
|
3 mcg
|
8
mcg
|
3 mcg
|
Fimbriae protein
(types 2 & 3)
|
0
|
5 mcg
|
0
|
5 mcg
|
Aluminum Phosphate
|
-
|
+
|
-
|
+
|
Formaldehyde
|
+
|
+
|
+
|
+
|
Polysorbate 80
|
+
|
-
|
+
|
-
|
Aluminum Hydroxide
|
+
|
-
|
+
|
-
|
Glutaraldehyde
|
-
|
+
|
-
|
+
|
Phenoxyethanol
|
-
|
+
|
-
|
+
|
Pregnancy Category †
|
C
|
C
|
-
|
-
|
* Flocculating units
† Risk not ruled out: "Animal reproduction
studies have shown an adverse effect on the fetus and there are no adequate and
well-controlled studies in humans, but potential benefits may warrant use of the
drug in pregnant women despite potential risks."
All information provided in the table comes
directly from the label for each vaccine. The label is a legal
document specifying the use of the product, ingredients, and other information,
which has been agreed through collaboration between the manufacturer and FDA/
Table created by Meryl Nass, MD
FIGURE 2. Annual incidence* of pertussis, by age group — United States, 1990–2016
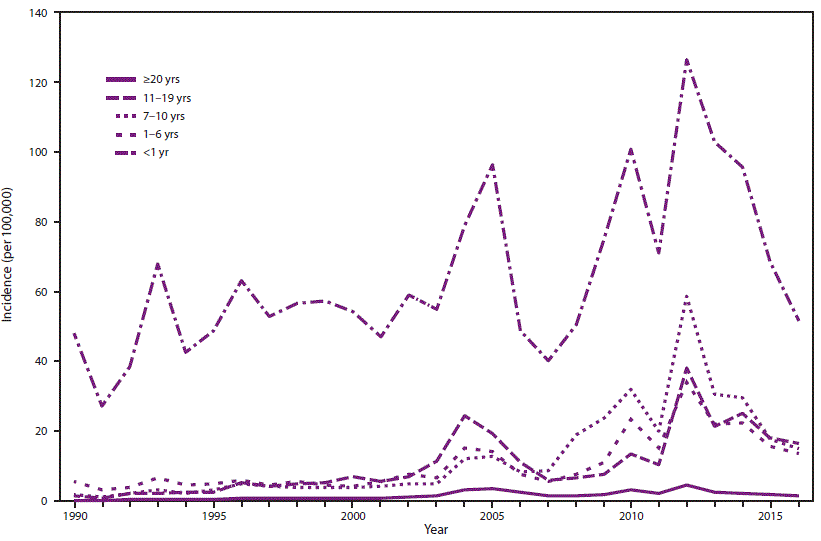
Sources: National Notifiable Diseases Surveillance System and Supplemental Pertussis Surveillance System.
* Per 100,000 population.
(The FDA removed that 2013 announcement in 2018; thanks to the reader who found it in the Wayback Machine.)
ReplyDeleteNews & Events
Home News & Events Newsroom Press Announcements
FDA NEWS RELEASE
For Immediate Release: Nov. 27, 2013
Media Inquiries: FDA- Jennifer Rodriguez, 301-796-8232, jennifer.rodriguez@fda.hhs.gov
NIH- Nalini Padmanabhan, 301-402-1663, padmanabhannm@niaid.nih.gov
Consumer Inquiries: 888-INFO-FDA,OCOD@fda.hhs.gov
FDA study helps provide an understanding of rising rates of whooping cough and response to vaccination
A new study is helping to provide a better understanding of vaccines for whooping cough, the common name for the disease pertussis. Based on an animal model, the study conducted by the U.S. Food and Drug Administration (FDA) and published November 25, 2013, in The Proceedings of the National Academy of Sciences, shows that acellular pertussis vaccines licensed by the FDA are effective in preventing the disease among those vaccinated, but suggests that they may not prevent infection from the bacteria that causes whooping cough in those vaccinated or its spread to other people, including those who may not be vaccinated.
Whooping cough rates in the United States have been increasing since the 1980s and reached a 50-year high in 2012. Whooping cough is a contagious respiratory disease caused by Bordetella pertussis bacteria. Initial symptoms include runny nose, sneezing, and a mild cough, which may seem like a typical cold. Usually, the cough slowly becomes more severe, and eventually the patient may experience bouts of rapid, violent coughing followed by the “whooping” sound that gives the disease its common name, when trying to take a breath. Whooping cough can cause serious and sometimes life-threatening complications, permanent disability, and even death, especially in infants and young children.
There are two types of pertussis vaccines, whole-cell and acellular. Whole-cell pertussis vaccines contain a whole-cell preparation, which means they contain killed, but complete, B. pertussis bacteria. The acellular pertussis vaccine is more purified and uses only selected portions of the pertussis bacteria to stimulate an immune response in an individual. In response to concerns about the side effects of the whole cell pertussis vaccine, acellular vaccines were developed and replaced the use of whole-cell pertussis vaccines in the U.S. and other countries in the 1990s; however, whole-cell pertussis vaccines are still used in many other countries.
“This study is critically important to understanding some of the reasons for the rising rates of pertussis and informing potential strategies to address this public health concern,” said Karen Midthun, M.D., director of the FDA’s Center for Biologics Evaluation and Research, where the study was conducted. “This research is a valuable contribution and brings us one step closer to understanding the problem. We are optimistic that more research on pertussis will lead to the identification of new and improved methods for preventing the disease.”
While the reasons for the increase in cases of whooping cough are not fully understood, multiple factors are likely involved, including diminished immunity from childhood pertussis vaccines, improved diagnostic testing, and increased reporting. With its own funds plus support from the National Institutes of Health (NIH), the FDA conducted the study to explore the possibility that acellular pertussis vaccines, while protecting against disease, might not prevent infection.
“There were 48,000 cases reported last year despite high rates of vaccination,” said Anthony S. Fauci, M.D., director of the NIH’s National Institute of Allergy and Infectious Diseases. “This resurgence suggests a need for research into the causes behind the increase in infections and improved ways to prevent the disease from spreading.”
https://wayback.archive-it.org/7993/20170111161009/http:/www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm376937.htm